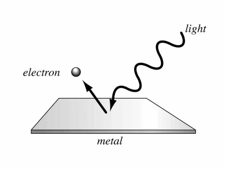
The Photoelectric Effect
· Electrons are emitted from the surface of a metal when electromagnetic radiation above a certain frequency is shone on it.
 |
· Wave model of light cannot explain this effect as in this theory more intense waves always carry more energy
· The photon theory of light is required, which describes light as small packets with energy proportional to their frequency – 1 ‘packet’ of energy is 1 quanta
· An electron is emitted from the surface when one electron in the metal absorbs one photon of light with enough energy to cause it to leave the surface
·
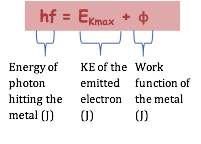 |
Any extra energy above this minimum gives the emitted photoelectron kinetic energy:
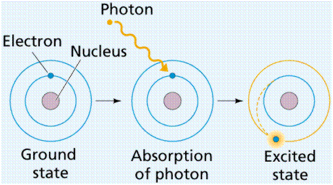
Electron Energy Levels in Atoms
· One consequence of quantum theory is that electrons must occupy specific energy levels in atoms
· The lowest energy level is called the ground state
· Excitation is when an electron moves up to a higher energy level – it requires energy to do this from either absorbing a photon or a collision with a free elctron
·
![]()
 |
 |
||
 |
 De-excitation is when an electron moves down to a lower energy level – it emits
energy as a photon of electromagnetic radiation
De-excitation is when an electron moves down to a lower energy level – it emits
energy as a photon of electromagnetic radiation
·
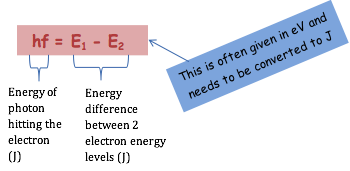 |
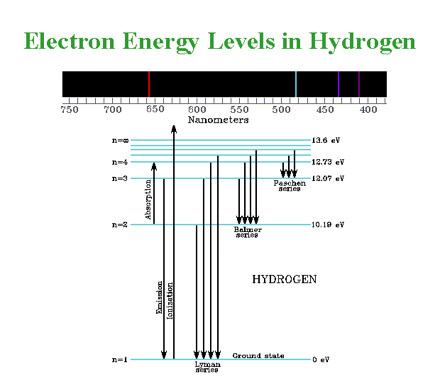
Excitation can occur when a photon hits an atom but only when the energy of the photon is greater than or equal to the energy level jump of the electron
Fluorescent Tubes
· Low density mercury vapour in a tube is ionized
· Electrons in the mercury are excited as they collide with one another and with free electrons
· The excited electrons then de-excite giving off photons with wavelengths in the UV part of the spectrum
· These UV photons hit the inside surface of the glass tube that is coated with a fluorescent material
· They are absorbed by electrons in the fluorescent material causing excitation
·
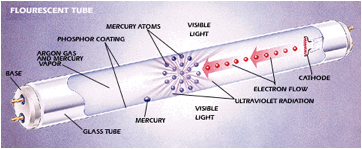 |
These electrons then de-excite indirectly giving off photons of visible light
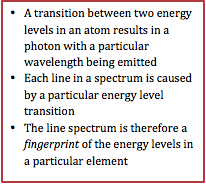
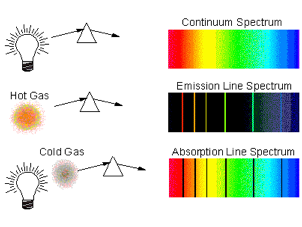
Spectra
![]()
![]()
![]()
![]()
Wave-Particle Duality
· Normally we think of light as a wave – this helps to explain phenomena like diffraction
· For effects like photo-electricity however this is not sufficient and it is useful to think of light as having a particle like nature which we call photons
· This thinking also works in reverse. If we take an object like an electron it is usual to think of it as a particle but it also has a wave like nature. E.g. diffraction can be done with a beam of electrons
· This dual nature of electromagnetic radiation and matter is called wave-particle duality
· The wavelength of matter can calculated as the De-Broglie wavelength, _
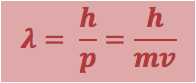 |
 |
To do:
Describe experiments to demonstrate the following:
- The wave nature of light
- The particle nature of light
- The wave nature of electrons

